How Many Ml of a .10 M Naoh
What volume of 0200 M NaOH will react with 500 mL of 0200 M aluminum nitrate AlNO 3 3. For more information please contact Centers for Disease Control and Prevention 1600 Clifton Road NE Atlanta GA 30333 Telephone 1- 800-CDC-INFO 232 -4636TTY.
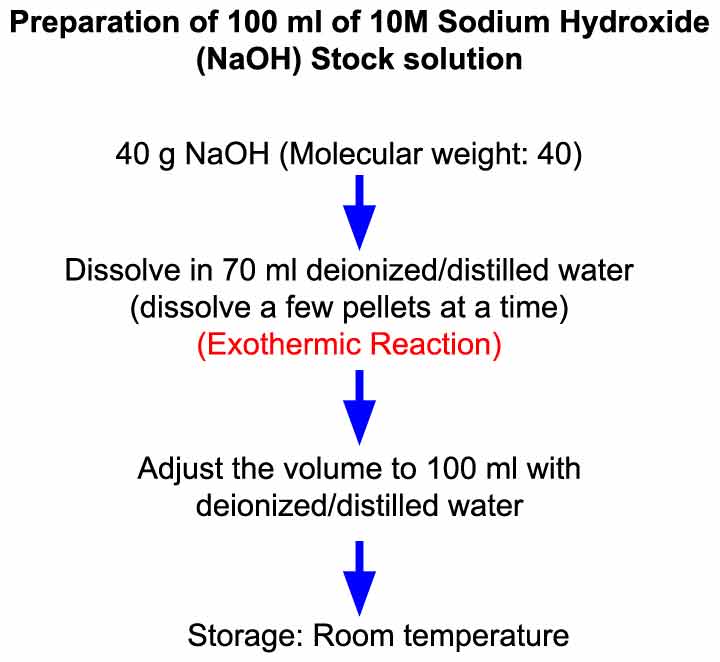
Preparation Of 10 M Sodium Hydroxide Naoh Solution Laboratory Notes
Colorbluemolarity moles of soluteliters of solution SImply put a 1-M solution will have 1 mole of solute dissolved in 1 liter of solution.

. 250 mL of a 095 M lead II chloride solution are mixed with 250 mL of a 075 M aluminum iodide solution. What mass of AlOH 3 will precipitate. Arrange these factors to calculate the.
Molarity is defined as moles of solute which in your case is sodium hydroxide NaOH divided by liters of solution. To prepare 100 ml of 10 M NaOH solution weigh out 40 g of NaOH molecular weight. Wash adult worms off plates into 15 mL conical tubes with M9.
If 257 ml of acid is required what is the concentration of the household ammonia. A 135 b 170 c 195 d 252 e 280 10. Spin down at 630 g for 30 s.
Now you know that your solution has a molarity of 0150 M and a volume of. Determine the maximum amount of solid lead II iodide molar mass 461008 gmol produced. How many grams of NaOH must be used to make 250 mL of a 1842 M solution of NaOH.
Youre titrating hydrochloric acid HCl a strong acid with sodium hydroxide NaOH a strong base so right from the start you should know that the pH at equivalence point must be equal to 7. Heres what I got. Which indicator identified by a letter could be used to titrate aqueous NH 3 with HCl solution.
To prepare roughly 1 M NaOH solution you must dissolve 20 g of NaOH in distilled water using 500 ml volumetric flask or 2 g of NaOH in distilled water using 50 ml volumetric flask. 200 mL of 08 M sodium carbonate solution. With a Pasteur pipette transfer the contents of the small vial to the stirring conical vial containing the vanillin and aqueous NaOH.
Use personal protective equipment lab coat gloves goggles etc for your safety and follow the guidelines of your institute. Hydrochloric acid and sodium hydroxide react in a 11 mole ratio to form water and aqueous sodium chloride HCl_textaq. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly.
Change moles of NaOH to. Group of answer choices 239 g 00238 g 109 g 130 g. For 6 mL of bleach solution add 45 mL of autoclaved H 2 O 360 μL of 50 NaOH and 12 mL of bleach.
Fill the tubes to 15 mL. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. 10 M NaOH solution.
Objective Preparation of 100 ml of 10 M NaOH solution in water. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Using the color-coded 10-mL syringe provided in the hood transfer 10-mL of a solution containing 1 M NaOH and NaBH4 80 mgmL to a clean small sample vial.
Calculate the pH of the solution resulting from the addition of 200 mL of 0100 M NaOH to 300 mL of 0100 M HNO 3. What is the concentration of a 100 ml sample of HCI if 355 ml of 0150 M NaOH is needed to titrate it to a pink endpoint. Start with what you know the final volume and concentration of the solution.
300 mL of 4 M sodium hydroxide solution. EXTREMELY LONG ANSWER. Aspirate supernatant down to 3 mL making sure not to disturb the pellet of worms.
A 100 ml sample of household ammonia NH3aq is titrated with 05 M HCI.

Solved 6 Calculate The Volume Of 1 M Naoh Needed To Prepare Chegg Com

When 100 Ml Of 1 M Naoh Solution Is Mixed With 10 Ml Of 10 M H 2 So 4 Youtube

Solved How Many Ml Of 0 10 M Naoh Should The Student Add To 20 Ml 0 10 M Hfor If She Wished To Prepare A Buffer With A Ph Of 3 55 The Same As
No comments for "How Many Ml of a .10 M Naoh"
Post a Comment